34+ Calculation Of Molality

Molality Definition Formula Calculation Video Lesson Transcript Study Com

An Aqueous Solution Is 34 H3p04 By Mass And Has Density 1 209 G Ml 1 Molarity I Molality Ii And Normality Iii Respectively Area Ab Bc Cd Dcorrect Answer Is Option A Can You Explain This
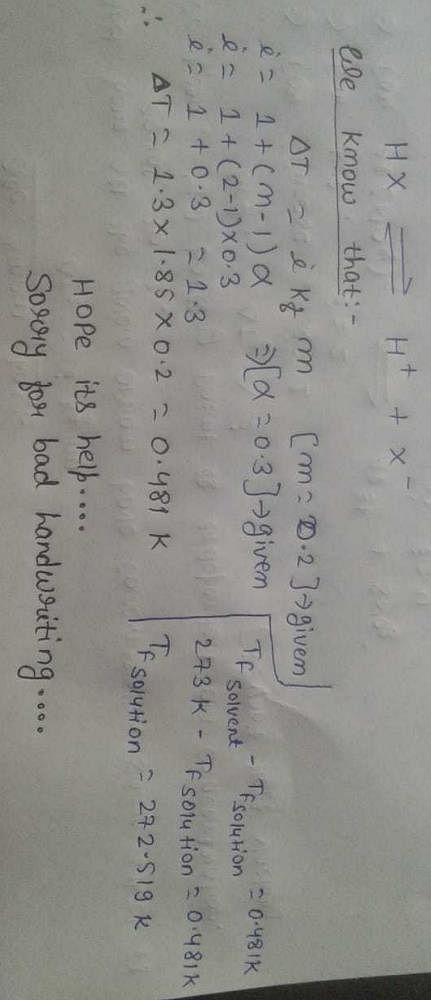
A Weak Acid Hx Have Dissociation Of Solution Is 0 3 And Molality Is 0 2 What Will Be The Freezing Point Of The Solution Kf 1 85 A 0 480 B 0 360 C 0 260 D 0 480
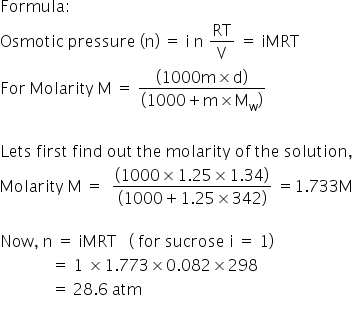
What Osmotic Pressure Would 1 25 Molal Sucrose Solution Exhibit At 25 Degree Celsius The Density Of Solution Is 1 34 Gram Per Ml 4r63rp99
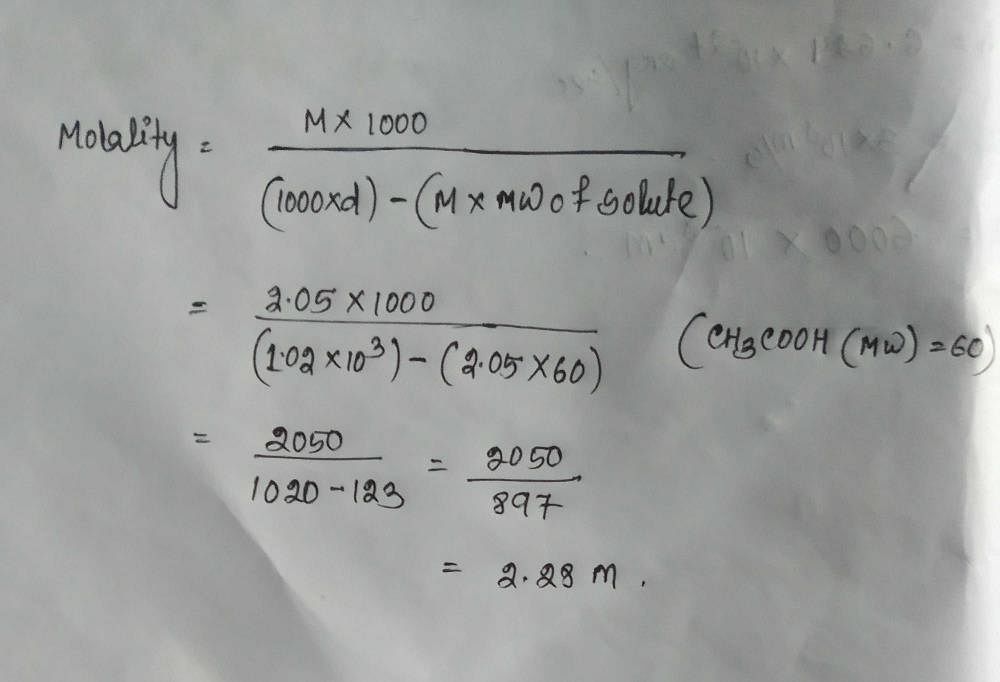
Density Of 2 0 5 M Solution Of Acetic Acid In Water Is 1 02 G Ml The Molality Of The Solution Is 1 0 44 Mol Kg 2 1 14 Mol Kg 3 3 28 Mol Kg 4 2 29 Mol Kg Edurev Neet Question

Percent Mass To Molality Youtube

Aleks Calculating Molality Youtube
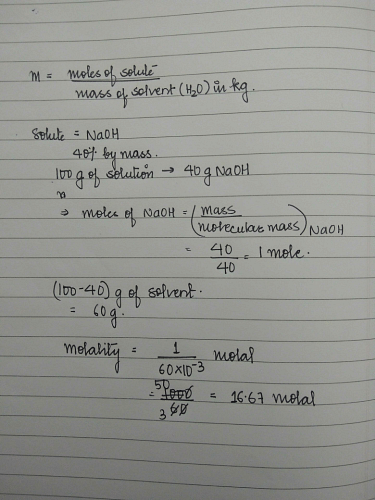
Calculate The Molality Of Naoh In Its Aqueous Solution Having Density 2g Ml And 40 Naoh By Mass Edurev Neet Question
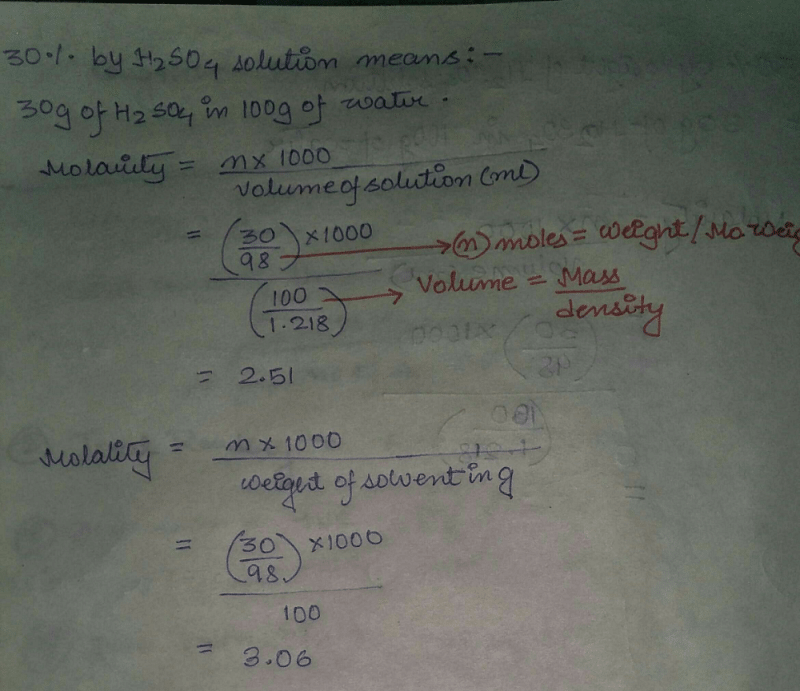
Calculate The Molarity And Molality Of The Solution Containing 30 By Weight Of H2so4 Density Of The Solution Is 1 218g Ml Edurev Class 11 Question

Oneclass Calculate Molality Of A 35 4 By Mass Aqueous Solution Of Phosphoric Acid H3po4 The Mo

Calculating Molality Using Density Youtube
An Aqueous Solution Is 34 H3p04 By Mass And Has Density 1 209 G Ml 1 Molarity I Molality Ii And Normality Iii Respectively Area Ab Bc Cd Dcorrect Answer Is Option A Can You Explain This

My Smart Class How To Calculate Molality
Molality It Is Defined As The Moles Of The Solute Present In 1kg Of The Solvent It Is Denoted By M Let Wa Grams Of The Solute Of Molecular Mass Ma Be

The Density Of 3m Solution Of Nacl Is 1 25g Ml 1 Calculate The Molality Of The Solution Edurev Class 11 Question

Calculating Molality Using Density Youtube

Molality Definition Formula Calculation Video Lesson Transcript Study Com
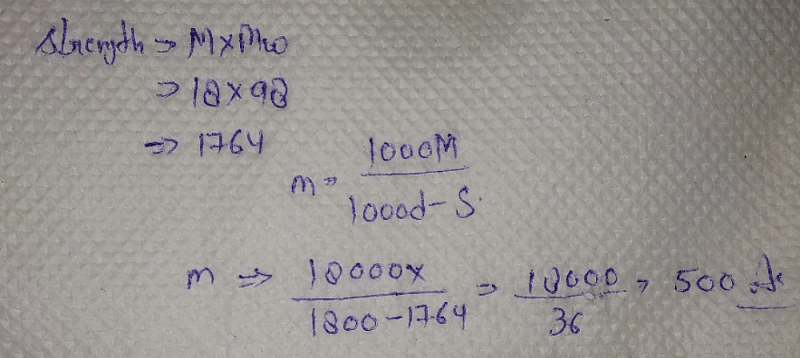
Molarity Of H2so4 Density 1 8 G Ml Is 18 M The Molality Of This H2so4 Will Bea 36b 200c 500d 18correct Answer Is Option C Can You Explain This Answer Edurev Question